Disruptive heart assist device by Procyrion
Procyrion, located in Houston, Texas was one of the laureates of 2015 Innovation Prize organized by Universal Biotech, subsidiary of Universal Medica Group. The start-up is developing Aortix™ a first-in-class circulatory assist device for ambulatory treatment of chronic heart failure. Heart failure affects over 6M people just in the US alone and around 600,000 new cases are diagnosed every year. The healthcare costs of treating patients with this indication is around $35B and they have very few therapeutic options. Out of the 6 M people diagnosed, less than 0.2% will eventually receive a heart transplant or a surgical Left Ventricular Assist Devices (LVAD). Aortix™ is a stand-alone device that is deployed during a trans-femoral catheter procedure. The device is placed in the descending thoracic aorta which enables the device to simultaneously unload (and rest) the heart and increase flow to the vital organs (e.g. the kidneys). This location has a number of additional unique benefits including eliminating the potential risks of thrombotic and aortic valve damage.
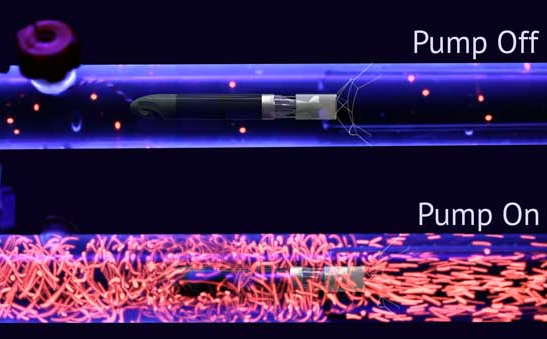
Benjamin Hertzog, CEO of Procyrion explains that the primary differentiator is the device used in an ambulatory patient. Primary indication of the device is for cardio-renal syndrome and Procyrion is exploring a number of other potential indications. The target segment for this market is patients with NHYA 3-4 heart failure (see below). The start-up is currently planning for its first human trials at the end of this year. Ben feels that the device has the potential to significantly reduce healthcare costs associated to patients with chronic heart failure by assisting the heart to function more efficiently and aiding it to heal. Patients with late-stage heart failure increase the healthcare costs due to a 1-in- 4 chance of them being readmitted to the hospital within the first 30 days of discharge. The vision of Procyrion team is to reduce readmission rate of late-stage heart failure patients by successfully completing their human trials and marketing the device to the said market. Ben is also exploring strategic fit to other indications and examining the market landscape of Aortix for healthier patients to prevent the progression of heart failure.
How heart failures are classified
Heart failures occure when the heart does not pump sufficiently to maintain blood flow to meet the body’s needs. The severity is usually the main criteria used to classify heart failures together with the level of limitation a patient is restricted to during physical activity. The most common classification system, New York Heart Association (NYHA) Functional Classification, described these classes as below:
| Class | Patient Symptoms |
| I | No limitation of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation, dyspnea (shortness of breath). |
| II | Slight limitation of physical activity. Comfortable at rest. Ordinary physical activity results in fatigue, palpitation, dyspnea (shortness of breath). |
| III | Marked limitation of physical activity. Comfortable at rest. Less than ordinary activity causes fatigue, palpitation, or dyspnea. |
| IV | Unable to carry on any physical activity without discomfort. Symptoms of heart failure at rest. If any physical activity is undertaken, discomfort increases. |
Sources:
www.procyrion.com
http://www.heart.org/heartorg
Disruptive heart assist device by Procyrion
Procyrion, located in Houston, Texas was one of the laureates of 2015 Innovation Prize organized by Universal Biotech, subsidiary of Universal Medica Group. The start-up is developing Aortix™ a first-in-class circulatory assist device for ambulatory treatment of chronic heart failure. Heart failure affects over 6M people just in the US alone and around 600,000 new cases are diagnosed every year. The healthcare costs of treating patients with this indication is around $35B and they have very few therapeutic options. Out of the 6 M people diagnosed, less than 0.2% will eventually receive a heart transplant or a surgical Left Ventricular Assist Devices (LVAD). Aortix™ is a stand-alone device that is deployed during a trans-femoral catheter procedure. The device is placed in the descending thoracic aorta which enables the device to simultaneously unload (and rest) the heart and increase flow to the vital organs (e.g. the kidneys). This location has a number of additional unique benefits including eliminating the potential risks of thrombotic and aortic valve damage.

Benjamin Hertzog, CEO of Procyrion explains that the primary differentiator is the device used in an ambulatory patient. Primary indication of the device is for cardio-renal syndrome and Procyrion is exploring a number of other potential indications. The target segment for this market is patients with NHYA 3-4 heart failure (see below). The start-up is currently planning for its first human trials at the end of this year. Ben feels that the device has the potential to significantly reduce healthcare costs associated to patients with chronic heart failure by assisting the heart to function more efficiently and aiding it to heal. Patients with late-stage heart failure increase the healthcare costs due to a 1-in- 4 chance of them being readmitted to the hospital within the first 30 days of discharge. The vision of Procyrion team is to reduce readmission rate of late-stage heart failure patients by successfully completing their human trials and marketing the device to the said market. Ben is also exploring strategic fit to other indications and examining the market landscape of Aortix for healthier patients to prevent the progression of heart failure.
How heart failures are classified
Heart failures occure when the heart does not pump sufficiently to maintain blood flow to meet the body’s needs. The severity is usually the main criteria used to classify heart failures together with the level of limitation a patient is restricted to during physical activity. The most common classification system, New York Heart Association (NYHA) Functional Classification, described these classes as below:
| Class | Patient Symptoms |
| I | No limitation of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation, dyspnea (shortness of breath). |
| II | Slight limitation of physical activity. Comfortable at rest. Ordinary physical activity results in fatigue, palpitation, dyspnea (shortness of breath). |
| III | Marked limitation of physical activity. Comfortable at rest. Less than ordinary activity causes fatigue, palpitation, or dyspnea. |
| IV | Unable to carry on any physical activity without discomfort. Symptoms of heart failure at rest. If any physical activity is undertaken, discomfort increases. |
Sources:
www.procyrion.com
http://www.heart.org/heartorg
